Understanding the Molecular Structure of HCOOH, CH₂, and H₂O
Scientists can explain the reaction of substances in chemical reactions, biological systems, and even in everyday life by being able to visualize the molecules at the atomic level. This article gives a detailed picture of the molecular structure of three main molecules: formic acid (HCOOH), a methylene group (CH₂), and water (H₂O). These are the ones which make a significant impact in both organic and inorganic chemistry. We will go over their atoms describe the way they are bonded, and discuss the effect of their geometry. This skill is vital for students, scientists and any person who is interested in the chemistry that influences our world. First of all let us analyze each molecule’s unique structure and properties one by one.
The Basics of Molecular Structure and Chemical Bonding
This is a very simple and basic explanation of molecular structure and its properties. The beginning of the article does briefly sketch the concept of molecular structure (atoms, bonds, and electron sharing), then it goes on to emphasize less known aspects of molecular geometry (reaction, polarity, physical properties). Atoms become interconnected via chemical bonds mostly covalent bonds where atoms share electrons.
The shape or geometry of a molecule is decided by the way these atoms and bonds are placed in space. The geometry of a molecule influences its reactivity polarity and physical properties. For instance, a bent structure (like in water) has different properties than a linear one. Knowing molecular structure is a good indicator how substances will behave in chemical reactions.
What is HCOOH (Formic Acid)?
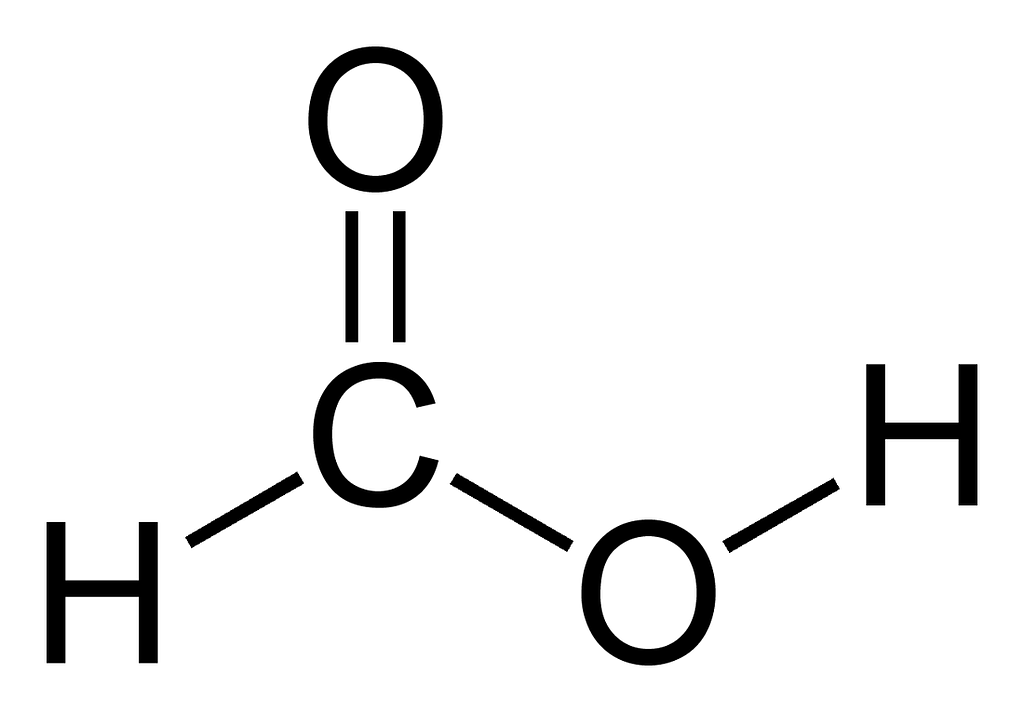
Formic acid, with the chemical formula HCOOH, is the simplest carboxylic acid. It consists of one carbon (C) atom bonded to two oxygen (O) atoms, one hydrogen (H) atom, and another H atom directly attached to the carboxyl group. The molecule has the following structure: H–C(=O)–OH. The carbon forms a double bond with one oxygen (making a carbonyl group) and a single bond with the OH group, making it a carboxylic acid. The arrangement forms a trigonal planar geometry around the central carbon atom due to the electron pairs. This structure allows formic acid to act as both a proton donor (acid) and a polar molecule, which makes it soluble in water and useful in chemical reactions.
Molecular Geometry and Polarity of HCOOH
Formic acid geometry greatly influenced its characteristics. The carbon atom in the carboxyl group –COOH was sp² hybridized which resulted in a planar triangular structure. The C=O double bond creates a high electron density region, whereas the O–H group facilitates hydrogen bonding with other molecules particularly water. This hydrogen bonding is one of the reasons for formic acid high boiling point and for its solubility in water. On top of that since the molecule has polar bonds and an uneven charge distribution, it is polar which means it will dissolve in other polar solvents. The same structural features make formic acid an ideal substance for use in the leather industry, as a preservative and in laboratory chemistry.
Exploring CH₂: The Methylene Group
Now let’s look at CH₂, known as the methylene group. This group is common in organic chemistry and often appears in molecules like methanol, ethylene, and alkanes. CH₂ consists of one carbon atom bonded to two hydrogen atoms. It usually appears as a part of a larger molecule because CH₂ by itself is a reactive intermediate. In such cases, carbon has two unpaired electrons, making it highly reactive and short-lived unless stabilized by bonding with other atoms. The geometry around the carbon in CH₂ depends on the bonding situation if it’s double-bonded to another atom (like in alkenes), it becomes planar due to sp² hybridization.
Structure and Hybridization of CH₂
The carbon atom in CH₂ is often sp² or sp³ hybridized, depending on the molecule it’s part of. For example, in ethylene (C₂H₄), CH₂ has sp² hybridization, with a trigonal planar geometry. Each hydrogen is bonded at approximately 120° angles, and the carbon shares a double bond with another carbon atom. However, in methane (CH₄), the CH₂ group would be sp³ hybridized, giving a tetrahedral geometry. These hybridization states influence the chemical behavior and bonding capabilities of the molecule. CH₂ groups are very important in the formation of polymers, fuels, and various synthetic chemicals. Their structural flexibility makes them fundamental in organic synthesis.
Understanding H₂O (Water) and Its Unique Structure
Water, with the formula H₂O, is one of the most essential molecules for life. Its structure includes two hydrogen atoms bonded to one oxygen atom. At first glance, it might seem like a simple linear molecule, but it actually has a bent or V-shaped geometry. This is due to the lone pairs of electrons on the oxygen atom. The oxygen in water is sp³ hybridized, which ideally gives a tetrahedral geometry, but two of those orbitals are filled with lone pairs. The bond angle is about 104.5°, not 109.5°, because the lone pairs push the hydrogen atoms closer together. This bent shape is crucial for water’s polarity and its unique properties.
Why Water is Polar and How That Affects Its Behavior
Water’s bent shape causes it to be a polar molecule. The oxygen atom is more electronegative than hydrogen, so it pulls electrons closer, giving the oxygen a partial negative charge and the hydrogens a partial positive charge. This polarity leads to hydrogen bonding between water molecules, where the hydrogen of one molecule is attracted to the oxygen of another. These interactions explain water’s high boiling point, surface tension, and excellent solvent properties. It can dissolve many ionic and polar substances, which is why water is often called the “universal solvent.” The molecular structure of H₂O plays a key role in biology, climate, and chemistry.
Comparing HCOOH, CH₂, and H₂O Structures
Now that we’ve explored each molecule, let’s compare their molecular structures and properties. HCOOH is a planar, polar molecule with acidic properties, used widely in industry. CH₂ is a building block in organic chemistry and appears in many molecules with different hybridizations. Water, on the other hand, is a bent molecule with strong polarity due to its lone pairs and plays a vital role in life processes. All three molecules are important, but they differ greatly in shape, polarity, and reactivity. Understanding these differences helps chemists predict reactions, develop new materials, and understand natural phenomena more deeply.
| Property | HCOOH (Formic Acid) | CH₂ (Methylene Group) | H₂O (Water) |
|---|---|---|---|
| Molecular Formula | HCOOH | CH₂ (usually part of larger molecules) | H₂O |
| Molecular Shape | Planar / Trigonal Planar | Varies (planar or tetrahedral) | Bent (V-shaped) |
| Hybridization | sp² (central carbon) | sp² or sp³ (depends on bonding) | sp³ (oxygen atom) |
| Polarity | Polar | Varies depending on the molecule | Strongly Polar |
| Bond Angles | ~120° | ~120° or ~109.5° | ~104.5° |
| Functional Group | Carboxylic Acid (-COOH) | Methylene Group | None (simple molecule) |
| Solubility in Water | High | Depends on full molecule | Complete solubility |
| Main Uses | Preservative, antibacterial, chemical synthesis | Building block in organic compounds | Universal solvent, essential for life |
| Reactivity | Acidic, participates in H-bonding | Highly reactive (unstable alone) | Stable, forms hydrogen bonds |
Applications of Understanding Molecular Structures
Why is knowing molecular structure so important? In fields like pharmaceuticals, biochemistry, and environmental science, understanding how molecules are built helps in designing drugs, developing treatments, and analyzing pollution. For instance, knowing that formic acid is polar and acidic helps in determining its usage in antibacterial products. CH₂ units are essential in making plastics and fuels, and understanding their bonding helps in synthetic chemistry. Water’s structure explains everything from why ice floats to how nutrients dissolve in the bloodstream. Molecular structure is not just an academic topic it’s at the heart of real-world chemical understanding.
Final Thoughts: Why Structure Determines Function
In chemistry, the phrase “structure determines function” holds great meaning. Whether it’s HCOOH, CH₂, or H₂O, each molecule’s unique arrangement of atoms governs how it behaves in different environments. From the acidity of formic acid to the reactivity of methylene and the life-supporting properties of water, molecular structures are the blueprint for chemical behavior. By understanding these structures, scientists and students alike gain a deeper appreciation for the materials that shape our world. In future studies, always consider the geometry, bonding, and polarity of a molecule—it might reveal more than you expect.
Frequently Asked Questions (FAQs)
The geometry of formic acid is mostly planar due to the sp² hybridization of the central carbon atom. This results in a trigonal planar structure with bond angles around 120°. The molecule contains a carboxylic acid group (-COOH) which makes it polar and reactive in acid-base reactions.
No, CH₂ (methylene) is typically unstable as a free molecule. It is commonly found as part of larger organic molecules. In those contexts, it may have sp² or sp³ hybridization, which affects its geometry and stability. On its own, CH₂ is highly reactive and exists only briefly as an intermediate in chemical reactions.
Water has a bent molecular shape because the oxygen atom contains two lone pairs of electrons. These lone pairs push the hydrogen atoms closer together, resulting in a bond angle of about 104.5°. This shape is responsible for water’s high polarity and hydrogen bonding behavior.
Formic acid is highly polar due to its carboxyl group (-COOH), which allows it to form hydrogen bonds with water molecules. This strong intermolecular interaction increases its solubility in water, making it useful in aqueous chemical reactions and biological systems.
The arrangement of atoms and types of chemical bonds determine how a molecule interacts with other substances. For example, water’s bent shape and polarity allow it to dissolve many substances, while formic acid’s acidic group allows it to donate protons. Structure influences reactivity, solubility, and physical behavior.
Yes. CH₂ units are fundamental building blocks in many polymers such as polyethylene. They act as repeating units in long molecular chains, and their ability to form stable covalent bonds with other CH₂ groups or atoms makes them ideal for plastic and synthetic material production.
Molecular structure helps chemists, biologists, and engineers predict chemical behavior and design effective materials. Whether you’re developing medications, purifying water, or designing synthetic fabrics, knowing how molecules are built is essential to making informed scientific decisions.
One thought on “Understanding the Molecular Structure of HCOOH, CH₂, and H₂O”
-
Pingback: HCOOH in Agriculture - HTB OFC3
Leave a Comment